
A cell made from magnesium and copper has a higher voltage than either of the other two combinations. The biggest voltage occurs when the difference in the reactivity of the two metals is the largest. Swapping the two electrodes means that the recorded voltage becomes negative. In the below table, the positive electrodes and what they are made from are listed along the top and the negative electrodes along the side.
Reactivity series chemistry gcse series#
If you are struggling to remember the reactivity series, this song (warning its quite catchy) might help you Reactivity Series song. If we connect different combinations of these metals to make a cell, we find that the voltage changes. Introduction into using the reactivity series and making predictions for chemical reactions. Here is a simple reactivity series: A simple reactivity series In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an external circuit is supplied. When this happens, we say the battery ‘goes flat’. In non-rechargeable cells, eg alkaline cells, a voltage is produced until one of the reactants is used up. 2.29 understand that metals can be arranged in a reactivity series based on the reactions of the metals and their compounds: potassium, sodium, lithium. GCSE CHEMISTRY METALS & THE REACTIVITY SERIES ANSWERS AND MARK SCHEMES QUESTIONSHEET 1 voltmeter 1 (b). A number of cells can be connected in series to make a battery, which has a higher voltage than a single cell. View metalsanswers.pdf from CHE MISC at IoBM. Chapters Reactivity Series of Metals - GCSE Chemistry KayScience 17.9K subscribers Subscribe 9.2K views 2 years ago In this video you will learn all the science for this. Understanding the reactivity series is fundamental to chemistry it explains why most reactions happen and what changes the particles will undergo during the reaction. The faster the reaction and the more energy released during that reaction the greater the reactivity of that metal element.A simple cell can be made by connecting two different metals in contact with an electrolyte. The reactivity series lists elements (mostly metals) in order of decreasing reactivity. The rate of reaction and the energy released during a reaction is directly related to the metal's reactivity. Metal elements which can lose electrons more easily than Hydrogen is able to lose its electron will react with water and with acids. The Reactivity Series can be found experimentally by observing the reactions between metals and either water or acid. The more easily a metal can lose electrons to become a metal ion the more reactive that metal is. When metals are placed in order of their reactivity it is called the Reactivity Series. The Reactivity Series is a list of metal elements in order of how easily they can lose electrons to form positive ions.Ībout The Reactivity Series The reactivity of a metal is determined by how easily it can lose electrons to form positive ions. By carrying out these reactions with lots of different elements, using lots of different conditions, scientists have been able put the. The metals potassium, sodium, lithium, calcium, magnesium. Elements above Carbon are found in minerals but they cannot be extracted with Carbon because Carbon is less reactive than those metals so we use electrolysis to extract them. This video explains how metals can be arranged in order of their reactivity in a reactivity series. More reactive metals displace less reactive metals from their compounds and react with water. Elements above Hydrogen but below Carbon are found in minerals, which are metal compounds, so they need to be extracted by using Carbon to displace the metal from the compound. Metals and reactivity series - (CCEA) The reactivity series ranks metals by how readily they react.


Elements below Hydrogen are found Native which means the metal element can be found not as part of a compound. Carbon and Hydrogen are important in the Reactivity Series as they can indicate how a metal can be extracted from a mineral.
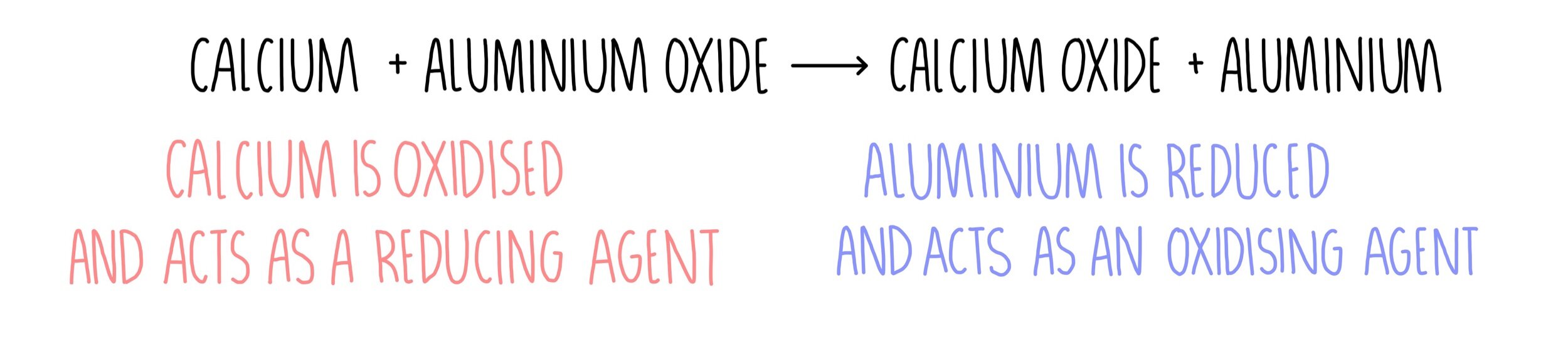
Elements higher on the reactivity series will displace those lower down on the reactivity series. The Reactivity Series is a list of elements in order of their reactivity.Ībout The Reactivity Series The Reactivity Series is used to predict the outcome of Displacement Reactions. The higher up the series a metal is, the more reactive the metal is and so the more stable the metal. The symbols and atomic numbers of some elements in the reactivity series. The stability of the metal carbonates can be related to the reactivity series of metals.


 0 kommentar(er)
0 kommentar(er)
